Table Of Content
- Understanding the role and importance of Design Reviews
- How To Elevate Device Security With A Custom Operating System
- Burgage plots and Regional Wealth are related
- Proving you designed correctly via Design Verification
- Maintaining a Design History File
- PMA Quality System
- Do You Need to Develop Your Product Under Design Controls?
- Step 16: Monitoring your design controls process
Once you have a building built, you have to hit the plus button to assign a family to work there. This takes one of the unassigned families and makes them dedicated to working at that building. They still tend the garden or livestock on their burgage plots and, more importantly, if you pause or remove the assignment from the building, they’ll go back to the unassigned pool.
Hyperquake Selects Fivestone For Interactive/Content For Johnson Controls' New OpenBlue Innovation Center - Live Design
Hyperquake Selects Fivestone For Interactive/Content For Johnson Controls' New OpenBlue Innovation Center.
Posted: Tue, 23 Jan 2024 08:00:00 GMT [source]
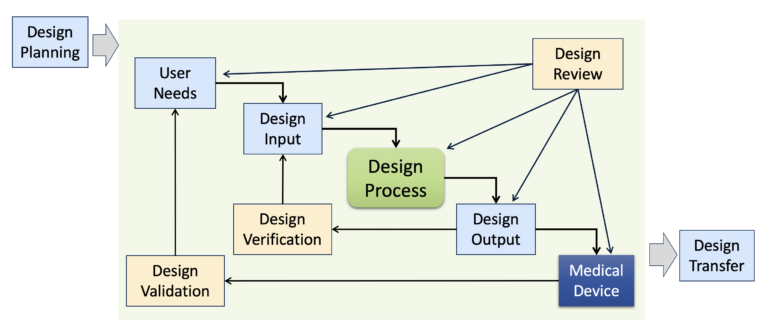
Understanding the role and importance of Design Reviews
During the process of Design Controls, your team will need to conduct formal design reviews and validate them. During these stages of Design Review, you will get the opportunity to evaluate the design requirements of your new medical device. Audit your design controls process to identify opportunities for improvement and preventive actions. Audits should include a review of the design process metrics, and you may consider establishing quality objectives for the improvement of the design process. This last step, and the standardization of design verification protocols in step five (5), are discussed in further detail in another blog by Medical Device Academy.
How To Elevate Device Security With A Custom Operating System
Polyamide fibers, commonly known as nylon, are the base for various textiles, including functional apparel, outdoor and sports gear. The two most common types of polyamides, PA 6 and PA 6.6, are difficult to identify and separate and are not collected by curbside recycling programs. Closing the loop on these materials is a crucial step towards a more sustainable future.
Burgage plots and Regional Wealth are related
The only required design review is a final design review to approve the commercial release of your product. All changes after that point should be under production controls, and changes should be documented in the (DMR)/Technical File (TF). If device modifications require a new 510k submission, then you should create a new design project and DHF for the device modification. The new DHF might have no changes to the user needs and design inputs, but you might have minor changes (e.g., a change in the sterilization method requires testing to revised design inputs). This document is intended to provide guidance to those involved in designing clinical studies intended to support pre-market submissions for medical devices and FDA staff who review those submissions. This guidance document describes different study design principles relevant to the development of medical device clinical studies that can be used to fulfill pre-market clinical data requirements.
Product development includes much, much more than just Design Controls. Things like budget, timeline, business development, marketing, sales, and so on. Your Risk Controls should align with and include Design Verification and Design Validation activities. And as your project progresses, you’ll soon find using these general-purpose tools take days, if not weeks, to properly update and maintain your traceability matrix. Yes, there are parts of the FDA regulations and ISO requirements that do apply to you, even if you are pre-market.
Let us say that your R&D department has come with a brilliant idea for a knee implant that will eliminate the disadvantages of currently available models. Now, you need to assure international regulatory agencies that your medical device will serve its purpose, is safe and efficacious before you market it. Production specifications typically consist of written documents such as assembly drawings, inspection and test specifications, and manufacturing instructions. However, they can also consist of electronic records, training materials such as video tapes or pictures, and manufacturing jigs and molds. Process validation may be conducted concurrently with design validation. Production devices used in design validation may have been manufactured in a production run during process validation.
Design Validation is about proving you designed the correct medical device. When you get to Design Verification, your goal is to prove your Design Outputs meet the Design Inputs. Design Verification is all about demonstrating you correctly designed your medical device. Remember Design Inputs are the roadmap used to design and develop your medical device. Design Inputs describe everything that is important and required about your medical device.
Do You Need to Develop Your Product Under Design Controls?
Confirm that acceptance criteria were established prior to the performance of verification and validation activities. Verify that the design outputs that are essential for the proper functioning of the device were identified. This plan needs to identify how many end users, what type of testing is required, and so on.
Firms may use a separate and less stringent change control procedure for pre-production design changes. Initial production units, lots, or batches, or their equivalents are to be used in design validation. Confirm that such production devices or their equivalents were used by reviewing the design validation documentation. If production devices were not used, the firm must demonstrate equivalency to production devices. Where there are differences, the manufacturer must justify why design validation results are valid for production units, lots or batches. The regulation is flexible and it does allow for the use of equivalent devices, but the burden is on the manufacturer to document that the units were indeed equivalent.

If you remember the discussion about User Needs, I shared that sometimes User Needs have a tendency to be a little ambiguous, using words like “easy” and “better”. With Design Validation, you need to figure out how to prove that your product accomplishes this. Although testing is not the only way to conduct Design Verification. You might also be able to employ inspection and analysis as acceptable methods for Design Verification.
Long Live Design Controls Navigating The Shift From QSR To QMSR - Med Device Online
Long Live Design Controls Navigating The Shift From QSR To QMSR.
Posted: Mon, 22 Apr 2024 04:05:15 GMT [source]
Ensuring Design Controls documentation and records are stored in a DHF is very important. When you evaluate risks, you will need to establish Risk Controls to mitigate and reduce risks. Design Outputs, Design Verifications, and Design Validations become these risk controls. Data collection and management designed for MedTech clinical trials. Design Validation, on the other hand, is in place to ensure that the final product can consistently meet all the User Needs determined in the first stage. The things seem to work, so now you really want to move forward with this product.
Each manufacturer shall establish and maintain procedures for verifying the device design. Design verification shall confirm that the design output meets the design input requirements. The results of the design verification, including identification of the design, method(s), the date, and the individual(s) performing the verification, shall be documented in the DHF.
You also need to ensure that you have all the necessary regulatory clearances. Outside the U.S. regulatory submissions and files, such as CE Mark Technical Files, are provided to regulatory bodies upon completion of Design Validation, during Design Transfer. FDA PMA devices will be ready for FDA review upon completion of Design Validation. Generally speaking, as you successfully finish Design Verification, this means you are approaching a time when you can prepare a regulatory submission.
Note, the v-model describes a product development methodology and approach. All about making sure you will be able to manufacture your medical device that you designed and developed. As noted above, Design Controls are all about ensuring the medical device you are developing is safe. Proof that you have designed a safe product that meets user needs and requirements. The first step of design control is determining User Needs as these will directly influence all subsequent parts (think of these as your main idea or your thesis of your essay). User Needs can come from various sources; however, a couple of key areas that must be clearly identified and determined are the intended use and indications for use.
We’re an online magazine dedicated to covering the best in international product design. We have a passion for the new, innovative, unique and undiscovered. At $495, the DaVinci Resolve Micro Color Panel is significantly more affordable than its larger counterparts.

No comments:
Post a Comment